Titanium dioxide is the most important white pigment. It has high refractive index, good chemical stability, weather resistance, whiteness, high color-removing power and hiding power, and is non-toxic and harmless. It has no stimulating effect on the human body and is widely used in coatings. , plastics, paper, ink, pharmaceuticals, fibers, cosmetics and other fields.
CAS No:13463-67-7 EINECS No.: 215-280-1 Molecular formula: TiO2 Molecular weight: 79.87 Density: 4.26 g/mL at 25 °C(lit.) Flash point: 2500-3000°C Melting point: 1840 °C Boiling point: 2900 °C
The chemical name of titanium dioxide: titanium dioxide, molecular formula TiO2, molecular weight 79.9, 59.95% of its mass composition is Ti, and O accounts for 40.05%.
Properties: Titanium dioxide is a soft, odorless and tasteless white powder with strong hiding power and coloring power. sulfuric acid. It turns yellow when heated, and turns white when cooled.
physical properties:
1. Relative density
Among the commonly used white pigments, the relative density of titanium dioxide is the smallest. Among white pigments of the same quality, titanium dioxide has the largest surface area and the highest pigment volume.
2. Melting point and boiling point
Since anatase transforms into rutile at high temperatures, the melting and boiling points of anatase titanium dioxide are practically non-existent. Only rutile titanium dioxide has a melting point and a boiling point. The melting point of rutile titanium dioxide is 1850°C, the melting point in air is (1830±15)°C, and the melting point in oxygen-rich environment is 1879°C. The melting point is related to the purity of titanium dioxide. The boiling point of rutile titanium dioxide is (3200±300)°C, and titanium dioxide is slightly volatile at this high temperature.
3. Dielectric constant
Titanium dioxide has excellent electrical properties due to its high dielectric constant. When determining some physical properties of titanium dioxide, it is necessary to consider the crystallization direction of titanium dioxide crystals. The dielectric constant of anatase titanium dioxide is relatively low, only 48.
4. Conductivity
Titanium dioxide has the properties of a semiconductor, its electrical conductivity increases rapidly with the rise of temperature, and it is also very sensitive to oxygen deficiency. The dielectric constant and semiconductor properties of rutile titanium dioxide are very important to the electronics industry, which can be used to produce electronic components such as ceramic capacitors.
5. Hardness
According to the Mohs scale of ten-tenths of hardness, the rutile titanium dioxide is 6-6.5, and the anatase titanium dioxide is 5.5-6.0. Therefore, anatase is used in chemical fiber extinction to avoid wear and tear on the spinneret hole.
6. Hygroscopicity
Although titanium dioxide is hydrophilic, its hygroscopicity is not too strong, and the rutile type is smaller than the anatase type. The hygroscopicity of titanium dioxide has a certain relationship with the size of its surface area. The large surface area and high hygroscopicity are also related to the surface treatment and properties.
7. Thermal stability
Titanium dioxide is a material with good thermal stability.
8. Granularity
The particle size distribution of titanium dioxide is a comprehensive index, which seriously affects the performance of titanium dioxide pigments and product application performance. Therefore, the discussion of hiding power and dispersibility can be directly analyzed from the particle size distribution.
The factors affecting the particle size distribution of titanium dioxide are more complicated. First, the size of the original particle size of hydrolysis. By controlling and adjusting the conditions of the hydrolysis process, the original particle size is within a certain range. The second is the calcination temperature. During the calcination process of metatitanic acid, the particles undergo a crystal transformation period and a growth period, and the appropriate temperature is controlled to make the growth of particles within a certain range. The last is the crushing of the product. Usually, the transformation of the Raymond mill and the adjustment of the analyzer speed are used to control the crushing quality. At the same time, other crushing equipment can be used, such as: universal mill, jet mill and hammer mill.
Crystal properties:
There are two stable crystal forms: rutile (Rutile-R type) and anatase (Anatase-A type). In general, R-type titanium dioxide has better weather resistance, water resistance and less yellowing characteristics, but the whiteness is slightly worse. Type A titanium dioxide has poor light resistance and weather resistance, but has better whiteness.
It can also be seen from the above figure that the titanium dioxide molecules in the rutile type on the left are relatively closely arranged, so it has greater stability and relative density, so it has a higher refractive index and dielectric constant and lower thermal conductivity. However, the anatase type can also be transformed into a rutile type at about 915°C.
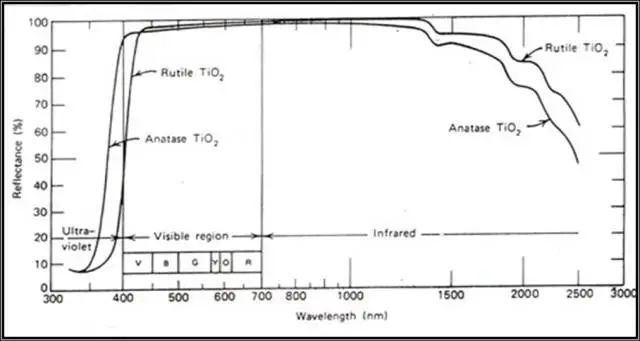
The rutile type absorbs more radiation energy than the anatase type at high energy (shorter wavelength). For rutile type titanium dioxide, in the UV-wavelength range (350-400nm) with strong lethality, its reflectivity to ultraviolet rays is much higher. It is lower than anatase titanium dioxide, because rutile titanium dioxide absorbs these ultraviolet rays. In this case, it will share much less ultraviolet light to the surrounding film-forming substances, resins, etc., so the service life of these organic substances will be longer. That is to say, why the so-called rutile titanium dioxide The reason why the weather resistance is better than the anatase type.
Reflection of anatase and rutile titanium dioxide in the ultraviolet, visible and infrared regions
Just because the rutile type absorbs more in the ultraviolet or near-ultraviolet region of visible light than the anatase type, that is to say, there is less blue light in the visible light range, so the rutile type titanium dioxide is slightly yellowish.
Although the anatase type also absorbs ultraviolet rays with shorter wavelengths other than visible light, it has little effect on the visible light seen by the human eye, so the color is bluish compared to the rutile type.
The rutile type is especially suitable for plastic products used outdoors, endowing the products with good light stability. The anatase type is mainly used for indoor products, but it has a slight blue light, high whiteness, high hiding power, strong tinting power and good dispersion.
Chemical properties:
The chemical properties of titanium dioxide are extremely stable, and it is a slightly acidic amphoteric oxide. It hardly reacts with other elements and compounds at room temperature, and has no effect on oxygen, ammonia, nitrogen, hydrogen sulfide, carbon dioxide, and sulfur dioxide. It is insoluble in water, fat, dilute acid, inorganic acid, and alkali, and only soluble in hydrogen. Hydrofluoric acid. However, under the action of light, titanium dioxide can undergo continuous redox reactions and has photochemical activity. This kind of photochemical activity is especially obvious in anatase titanium dioxide under ultraviolet irradiation. This property makes titanium dioxide not only a photosensitive oxidation catalyst for some inorganic compounds, but also a photosensitive reduction catalyst for some organic compounds.
Emergency treatment: isolate the leaked contaminated area and restrict access. It is recommended that emergency personnel wear dust masks (full face masks) and general work clothes. Avoid raising dust, sweep it up carefully, put it in a bag and transfer it to a safe place. If a large amount of leakage occurs, cover it with plastic sheeting or canvas. Collect and recycle or transport to waste disposal site for disposal.
Titanium dioxide (or titanium dioxide) is widely used in various structural surface coatings, paper coatings and fillers, plastics and elastomers, and other uses include ceramics, glass, catalysts, coated fabrics, printing inks, roofing granules and fluxes. According to statistics, the global demand for titanium dioxide in 2006 reached 4.6 million tons, of which the paint industry accounted for 58%, the plastic industry accounted for 23%, papermaking 10%, and other 9%. Titanium dioxide can be produced from ilmenite, rutile, or titanium slag. There are two kinds of titanium dioxide production processes: the sulfate process and the chloride process. The technology of the sulfate process is simpler than that of the chloride process, and low-grade and relatively cheap minerals can be used. Today, about 47% of the world's production capacity uses the sulfate process, and 53% of the production capacity is the chloride process.
Post time: Jun-05-2023